영형
2025년 8월 1일, 유럽연합 사법재판소는 2022년 판결을 지지하는 최종 판결을 내렸습니다.
이는 이산화티타늄(TiO₂) 분말의 발암성 분류를 해제하는 것을 의미합니다. 이 결정은 전 세계 수많은 산업에 영향을 미쳤던 거의 10년간의 과학적 및 규제적 논쟁에 종지부를 찍는 것을 의미합니다.
이산화티타늄
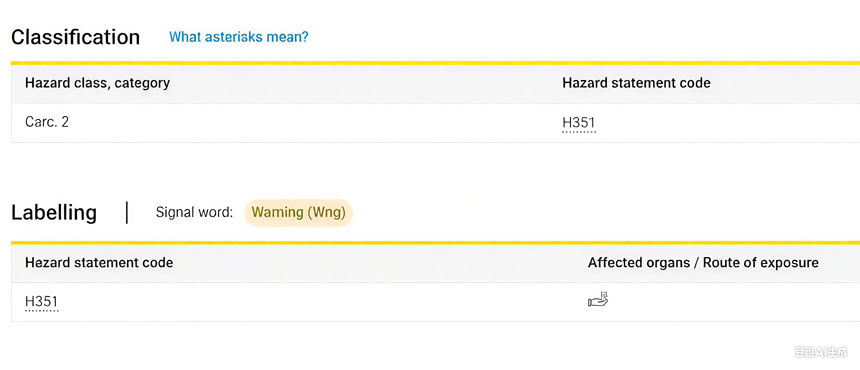
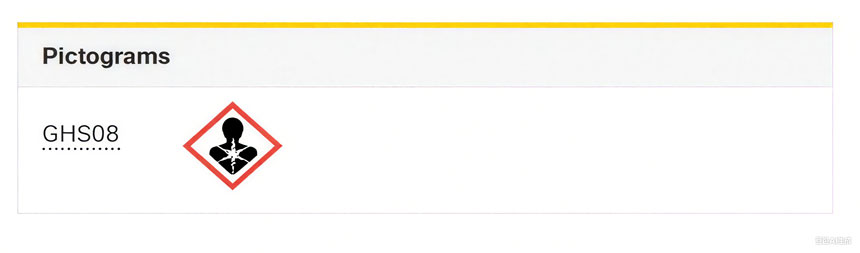
TiO2 분말은 뛰어난 은폐력과 백색도로 코팅, 의약품, 식품 등에 널리 사용됩니다. 2016년 프랑스 식품안전환경산업보건청(ANSES)은 유럽화학물질청(ECHA)에 분말 형태의 이산화티타늄을 "흡입 발암물질"로 분류해 줄 것을 요청하는 제안서를 제출했습니다. 2017년 ECHA의 위해성평가위원회(RAC)는 이산화티타늄을 "의심 발암물질 2등급"으로 분류하는 의견을 채택했습니다. 2019년 10월, 유럽 집행위원회는 위임 규정(DE) 2020/217을 채택하여 분말 형태의 이산화티타늄(입자 크기 1% 이상 10마이크론 이하)을 의심 발암물질 2등급으로 공식 분류하고 "H351: 흡입 시 암을 유발할 수 있음"이라는 경고 라벨 사용을 의무화했습니다.
현재의 분류 및 라벨링은 다음과 같습니다.
이산화티타늄 분류 타임라인
|
년도
|
이벤트
|
영향
|
|
2016
|
프랑스의 ANSES는 TiO₂를 다음과 같이 분류할 것을 제안합니다.
발암 물질
|
초기 규제 우려 제기
|
|
2017
|
ECHA의 RAC는 2등급 분류를 지원합니다.
|
과학위원회 승인
|
|
2019
|
EU 위원회, 위임 규정(EU) 2020/217 채택
|
의무적 H351 경고 라벨 시행
|
|
2022
|
EU 일반법원이 분류를 취소하다
|
법적 도전 성공
|
|
2025
|
EU 사법재판소, 취소 확정
|
분쟁의 최종 해결
|
결정의 과학적 근거
CJEU의 판결은 세 가지 주요 과학적 고려 사항에 근거했습니다.
1. 작용 기전:
법원은 쥐 연구에서 관찰된 폐 종양이 TiO₂ 자체의 내재적 발암성보다는 입자 과부하로 인해 발생했다는 데 동의했습니다.
2. 인간에 대한 외삽법:
법원은 쥐 흡입 연구가 특히 일반적인 노출 수준에서 인간의 암 위험을 안정적으로 예측할 수 있다는 것을 입증할 만한 증거가 충분하지 않다고 결론지었습니다.
3. 기타 설명:
판결문에서는 관찰된 효과가 화학적 독성보다는 입자 축적으로 인한 염증 때문일 수 있다고 밝혔다.
직접적인 산업 영향
이번 결정은 여러 산업에 상당한 구제책을 제공할 것입니다.
페인트 및 코팅
:
대부분의 건축 및 산업용 코팅에 있는 경고 라벨을 제거하세요
플라스틱
:
식품 포장 및 소비재에 대한 분류 부담을 줄여줍니다.
화장품:
자외선 차단제와 컬러 화장품은 이제 발암물질 경고 없이 판매될 수 있습니다.
식품 산업:
식품 응용 분야에서 TiO₂(E171) 사용에 대한 신뢰 회복
글로벌 규제 영향
EU의 결정은 회원국에 구속력이 있지만, 다른 지역에서는 다르게 반응할 수도 있습니다.
우리를:
직업 안전 및 건강 관리국(OSHA)은 현재의 비발암성 분류를 유지합니다.
캐나다:
캐나다 보건부는 새로운 증거를 계속 모니터링하고 있습니다.
아시아:
대부분의 시장은 EU의 예방 분류를 채택한 적이 없습니다.
이산화티타늄을 함유한 제품
TiO₂는 여전히 많은 일반 제품에 필수적입니다.
|
제품 카테고리
|
TiO₂ 기능
|
일반적인 농도
|
|
그림 물감
|
불투명제/흰색
너
|
15-25%
|
|
플라스틱
|
자외선 안정제
|
0.5-5%
|
|
자외선 차단제
|
UV 필터
|
2-10%
|
|
식품(E171)
|
착색제
|
0.1-1%
|
|
종이
|
브라이트너
|
2-8%
|
EU 사법재판소의 최종 판결은 이산화티타늄의 안전성에 대해 절실히 필요했던 명확성을 제공하며, 많은 과학자와 제조업체의 입장을 확인시켜 주었습니다. 규제적 경계는 여전히 중요하지만, 이번 판결은 업계가 불필요한 발암성 경고 없이 이산화티타늄의 귀중한 특성을 활용할 수 있도록 해줍니다.
자주 묻는 질문
Q1: 이산화티타늄 TiO2 분말이란 무엇입니까?
A: 이산화티타늄은 다양한 산업 및 소비자 분야에서 백색 안료와 기능성 첨가제로 사용되는 천연적으로 발생하는 이산화티타늄입니다.
Q2: 일반적인 안료에 이산화티타늄이 함유되어 있나요?
A: 몇 가지 중요한 안료에는 이산화티타늄이 포함되어 있습니다.
이산화티타늄(TiO₂): 순수 이산화티타늄 안료
진주광택 안료
: 종종 이산화티타늄 코팅 마이카는 무지개빛 효과를 얻는 데 사용됩니다.
복합 안료: 많은 안료는 이산화티타늄을 다른 착색제와 결합합니다.
질문 3: 이산화티타늄을 화장품에 사용해도 안전한가요?
A: 의도된 용도로는 안전합니다. EU의 한 판결은 피부 접촉을 통해 암을 유발한다는 증거가 없음을 명확히 확인했습니다.
Q4: 식품 등급 이산화티타늄(E171)이 EU에서 재승인될까요?
A: 법원 판결이 E171을 자동으로 복원하지는 않지만, 2022년 금지에 대한 과학적 근거를 제거했기 때문에 재고의 여지가 생길 수 있습니다.
Q5: 제조업체는 제품 라벨을 어떻게 업데이트해야 합니까?
에이:
1. 회사는: 이산화티타늄이 함유된 제품에서 H351 경고를 즉시 제거해야 합니다.
2. 안전 데이터 시트를 검토하여 규정 준수 사항을 업데이트하세요.
3. 과도기적 라벨링 요구 사항에 대해서는 법률 팀과 상의하세요.
화학 위험 평가 분야 15년 경력의 재료 안전 전문가 리사 첸 박사가 작성했습니다. 최종 업데이트: 2025년 8월










 +86 13965049124
+86 13965049124
 한국의
한국의  English
English français
français русский
русский italiano
italiano español
español português
português العربية
العربية ไทย
ไทย Tiếng Việt
Tiếng Việt







